|
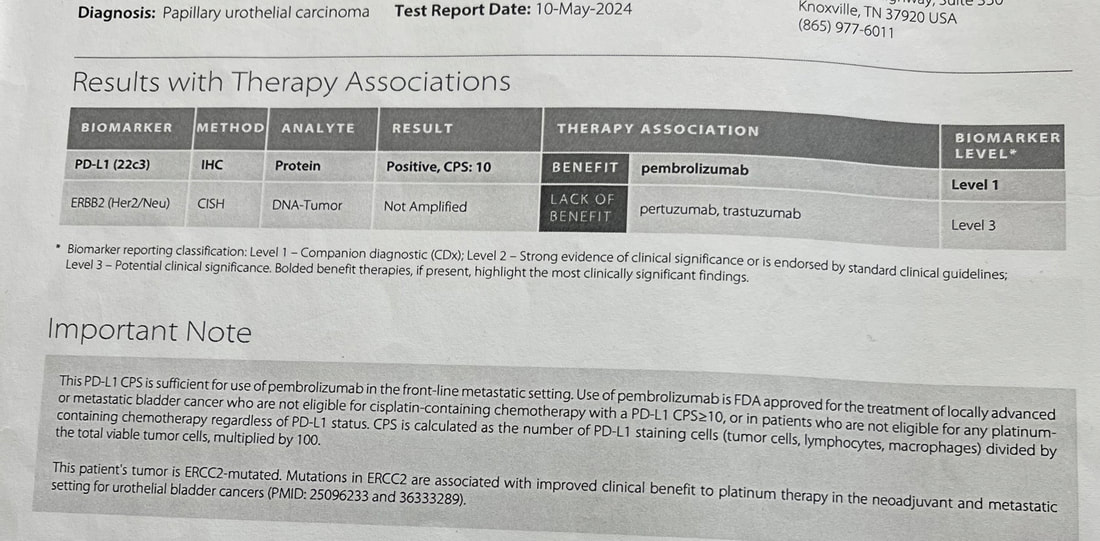
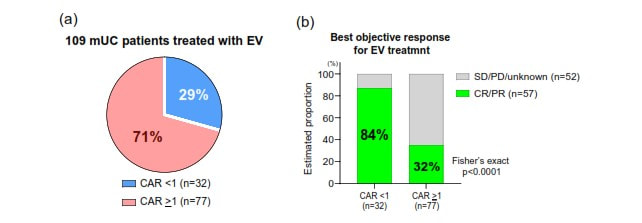
1 year and 6 days ago I had my first PET/CT scan . [I've mentioned that I led software development for the first generation of PET/CT systems - but this was my first image as a patient.] That scan showed locoregional spread of my muscle invasive bladder cancer (MIBC) to nearby lymph nodes and changed the standard of care options - and the options would change again 35 days later with the FDA approval of enfortumab vedotin + pembrolizumab (EV/pembo - Padcev/Keytruda) as a first line option for locally advanced and metastatic MIBC. The performance in the phase 3 clinical trial (EV 302) that lead to approval was impressive - overall response rate of 67.7% and complete response rate of 29.1%. Testing was done looking at Nectin 4 expression (which enfortumab vedotin targets) and PD-L1 (which pembrolizmab targets) and both high and low groups showed had benefits in progression free and overall survival - therefore, no testing is required to begin EV/pembro treatment for locally advanced and metastatic MIBC. As I was seeing a good response - I was still curous if there were any explanation (beyond the power of prayer and God's grace) as to why. I requested a genetic analyis and received the report from a sample of my tumor resected 217 days earier. The report showed that I had a combined positive score (CPS) of 10 - which according to one study shows that I was likely to receive benefit from pembrolizumab. My tumor did not have FGFR mutations for which an FGFR inhibitor such as erdafitinib would be effective. A Nectin-4 histology score (H-score) could evalute its expression in my tumor cells. However, another study showed that a more common blood marker - C-reactive protein to albumin ratio (CAR) - can predict objective response to EV. The results showed that lower CAR is associated with a better response. I am familiar with C-reactive protein (CRP) from a previous health journey (see earlier in the blog) where I lost 100 lbs and reversed type 2 diabetes. CRP is an indicator of inflammation - which is associated with type 2 diabetes. It may be that maintaing my weight, exercising and maintaining a low carb real food diet (including healthy fats and proteins) has resulted in a CAR <1 and contributed to a good response to EV. My lab work before each infusion includes an albumin measurement - I will ask to see if CRP can be measured as well to see if my CAR is below 1.
0 Comments
In conversation with my oncologist I was made aware that there are clinical studies which show that a probiotic could enhance the performance of immune checkpoint inhibitors - which was of particular interest as I was now taking pembrolizumab (Keytruda) alone.
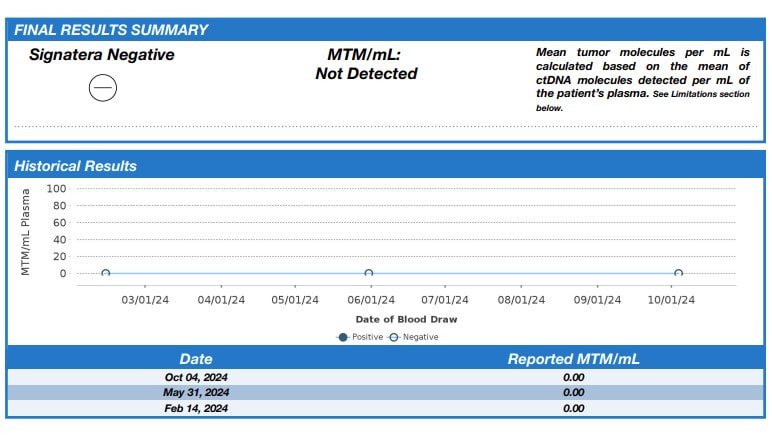
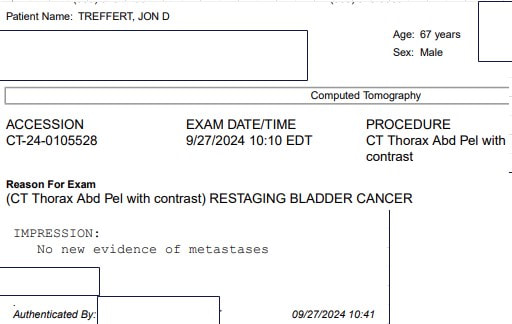
The probiotic used in the studies is Clostridium butyricum MIYAIRA 588 - a specific strain of anaerobic, butyric acid forming, Gram-positive bacterium isolated from a soil sample in Japan. Its primary use as a supplement is to regulate gut health (see the label above from the product I ordered from a supplier that imports from Japan). As documented earlier in my blog - 7 years ago, I began a low carb/high fat diet which, along with green exercise and intermittent and periodic prolonged fasting, was key to losing 100 lbs and reversing type 2 diabetes. I have maintained my weight and blood glucose by continuing to follow this regimen. CBM 588 is believed to modulate some potential gastroinstestinal side effects of a ketogenic diet, increase insulin sensitivity and optimize lipid metabolism. While more work needs to be done, in addition to bowel regulation, CBM 588 appears to be a good addition to my diet. What is most impressive for my current health journey are the results being reported when CBM 588 is used with immune checkpoint inibitors - specifically for renal and non-small cell lung cancer. The gut biome is known to play a key role in regulating the immune system. Imbalances, known as dysbiosis can lead to immune disfunction. In a phase 1b trial for renal cancer - the objective response rate (ORR) was 58% for those receiving immunotherapy and CBM 588, compared to 20% for those receiving immunotherapy alone. The immunotherapy was a combination of nivolumab (Opdivo) and ipilimumab (Yervoy). Nivolumab is a PD-1 checkpoint inhibitor like pembrolizumab (Keytruda). Ipilimumab targets the CTLA-4 receptor on T-cells. The improvements are consistent with the hypothesis that supplementation with CBM 588 works to reduce dysbiosis, support the immune system and improve efficacy of immune checkpoint inhibitors. A phase 1b trial examines safety - e.g. dosage and pharmokinetics. A phase 2 trial is planned to look at efficacy (as well as further examination of safety and optimal dosing). In the lung cancer study cited - 75% of the patients had a combination of chemotherapy and pembrolizumab. The addition of CBM 588 was associated with a longer overall survival and progression free survival. Less relevant for me, but interesting nonetheless - CBM 588 also been shown to be as effective against (non-muscle invasive) bladder cancer cells and potentially safer than the first immunotherapy BCG in in-vitro and in-vivo laboratory studies. I found a supplier of Strong Miyarisan, a supplement supplying CBM 588 on eBay (see label above). Each tablet contains 30 mg CBM 588. The recommended adult dose is 3 tablets 3 times per day or 270 mg. The dose administered in the renal cancer trial was 80 mg twice per day or 160 mg. I would welcome (and will lobby as I can for) a clinical trial of patients beginning a first line treatment of Padcev + Keytruda for locally advanced or metastatic muscle invasive bladder cancer - one arm supplemented with CBM 588 - the other without. This is the pathway that would allow doctors to prescribe CBM 588 as part of treatment. In the meantime, I am proceeding with the assumption that CBM 588 may boost efficacy of pembrolizumab. I am going to start with 2 tablets 3 times per day or 180 mg. As this supplement is considered safe, I believe that at a minimum I will have good intestinal health, likely benefits for my ketogenic diet (including insulin sensitivity and improved bowel health) and potentially improved efficacy of Keytruda fighting any cancer that remains. I will report any adverse effects. I currently have no evidence of disease by imaging or circulating tumor DNA and I am just one patient so it will be difficult to know if the addition of CBM 588 has been effective (or harmful) - I will continue to monitor any relevant trials. As with any decision affecting treatment - patients should discuss with their care team. I received my third negative circulating tumor DNA (Signatera) test today. I discovered my disease - which turned out to be locally advanced muscle invasive bladder cancer (MIBC) - 390 days ago. I had a transurethral resection of the bladder tumor (TURBT) 15 days later and began treatment with enfortumab vedotin + pembrolizumab (EV/pembro or Padcev/Keytruda) 89 days after discovering the disease. The tumor sample was used by Natera to design my Signatera test to find evidence of residual disease by detecting tumor DNA shed into the blood from anywhere in the body. 3 months after my TURBT, 1 month after beginning treatment, I underwent an endoscopic procedure to remove and possibly replace a stent in my right ureter placed during the TURBT due to the tumor obstructing flow from my right kidney. The kidney flow was now brisk and there was no evidence of my primary tumor in the bladder. 84 days after beginning treatment (4 cycles) PET/CT now showed no significant uptake in my previously active pelvic lymph nodes or evidence of new metastatic disease. Two follow-up CT scans with contrast would also show no new significant findings. Given the good results, due to low grade adverse effects, we suspended the Padcev component after 7 cycles and have continued with Keytruda alone (currently 7 more cycles). Sensation in my fingertips and toes, sense of taste and hair have since returned. 29% of the participants in the EV 302 trial (that led to the accelerated approval of EV/pembro as a first line treatment for patients with locally advanced or metastatic muscle invasive bladder cancer) had a complete pathological response. I have no plans to pursue removal of or chemoradiation to the bladder. I am working to see that more high responders are followed to see how durable the response is. In the meantiume I hope to encourage more patients try EV/pembro as their first line treatment before pursuing radical cystectomy or bladder sparing with trimodality therapy. The vision was articulated by a leader in the field - Dr. Shilpa Gupta - at the recent EMSO 2024 meeting I began my 7th cycle of enfortumab vedotin and pembrolizumab (Padcev+Keytruda) on May 31. I continued with pembrolizumab only on June 21. On September 13 I began my 6th cycle of pembrolizumab only. This is my second follow-up using CT with contrast since changing to immunotherapy alone. I would like to schedule a PET/CT and/or circulating tumor DNA to confirm - but I am happy to see a report of no evidence of recurrence or new disease by CT.
My cancer journey is documented on this blog. After over 30 years of leading software development of medical devices for the detection and treatment of cancer, I've had the opportunity to view the state of the industries I've contributed to as a member of the population they exist to serve. I've also begun work as a patient advocate and have had a chance to meet many fine folks who have walked or are walking through a bladder cancer (or other cancer) journeys. I now have a series of recommendations and new causes to pursue:
Educate and advocate for PET/CT staging of advanced muscle invasive bladder cancer After transurethral resection of my bladder tumor (TURBT), pathology confirmed it as invasive high grade papillary urothelial cell carcinoma with musclaris propria present. Anatomic imaging - CT and MRI, with and without contrast, were ordered for staging. No incidental findings were observed. I then requested a PET/CT. A whole-body PET/CT scan found locoregional spread to left presacral and left pelvic lymph nodes. This significantly changed the standard of care guidance for my case. Review of the current data demonstrates the promise of PET/CT relative to CT and MR for detection of lymph node or metastatic involvement in muscle invasive bladder cancer (MIBC). Standardization of the process may lead to wider adoption and reimbursement and reduced understaging of MIBC. Educate and advocate for bladder sparing treatment with trimodality therapy (particularly with protons) A majority of patients with muscle invasive bladder cancer undergo radical cystectomy (surgical removal of bladder, lymph nodes and often other nearby organs) and a urinary diversion: ileal conduit (incontinent diversion), neobladder(continent diversion) or continent cutaneous diversion. There remains a significant rate of recurrence with radical cystectomy (RC). There may also be neoadjuvant or adjuvant chemotherapy to minimize microscopic disease. Trimodality therapy is a bladder sparing option which employs radiation therapy and concurrent chemotherapy following TURBT. Having lead software development for a new proton therapy system, I had intended to pursue trimodality therapy with radiation delivered by a proton therapy system. I had access at a facility 20 minutes away in Knoxville or with the system I had lead software development for in Franklin Tennessee (where my sister now lives). I was initially disappointed to see that bladder cancer was not listed as an indication on the web site of either facility. After inquiring, I was happy to learn that a limited number of bladder cancers and lymph nodes had been treated at both facilities. A recent publication concluded that their "multi-institutional study provides the best evidence to date showing similar oncological outcomes between radical cystectomy and trimodality therapy for select patients with muscle-invasive bladder cancer. These results support that trimodality therapy, in the setting of multidisciplinary shared decision-making, should be offered to all suitable candidates with muscle-invasive bladder cancer and not only to patients with significant comorbidities for whom surgery is not an option.” I believe that proton therapy provides improved sparing of health tissue and quality of life and had initiated a consultation. A recent review comparing proton and x-ray therapy in MIBC showed improved overall survival with proton therapy - calling for more study and follow up. Access and reimbursement remains a challenge for the proton therapy industry and trimodality therapy for bladder sparing seems to me to be a significant opportunity to collaborate in demonstrating its benefits. Educate and advocate for potential bladder sparing with EV/pembro I knew that in Tennesse, my best route for approval of proton therapy was through Medicare. On learning of my diagnosis I resigned my job, left my employer insurance and applied for Medicare Part B (I was 66). My application was delayed (although I had sent in all the required forms) and while I was waiting, I read of the practice changing results presented for enfortumab vedotin and pembrolizumab (EV/Pembro) in the EV-302 phase III trial. My Medicare was approved on Dec 1, 2023 (retroactive to Nov 1, 2023). I began treatment with EV/pembro on Dec 11, 2023. On Dec 15, the FDA granted accelerated approval of EV/pembro as a first line option for locally advanced or metastatic urothelial cell cancer. 29% of the participants in the EV-302 trial had a complete pathological response. The details are presented in this blog - I currently have no evidence of disease by imaging or circulating tumor DNA and at present have no plans to pursue chemoradiation or surgery. More data needs to assembled following high responders to EV/pembro to quantify durability of the response to see if trimodality or RC can be avoided. As part of this activity, guidance for dose de-escalation for high responders to EV/pembro needs to be generated. Advance biomarker development to predict response to EV/pembro EV/pembro is an all comers treatment not requiring biomarker tests for inclusion. Tests exist today to evaluate expression of PD-L1 to predict response to pembrolizumab. I did have genetic testing done (after beginning treatment) which showed I have a PD-L1 CPS score of 10 - indicating likelihood of benefit to pembrolizumab. Enfortumab vedotin is an antibody drug conjugate which binds to an immunoglobulin like protein Nectin-4. Nectin-4 is overexpressed in 97% of bladder cancers. The diagnostic and prognostic potential of Nectin-4 is currently being explored. A PET radiotracer (68)Ga-N188 has been developed for imaging of Nectin-4 and may offer a tool for diagnosing bladder cancer and selecting patients for treatment with/predicting response to enfortumab vedotin. It may also be possible someday to engineer theranostic pairs employing alpha and beta emitting radionuclides similar to those available today for prostate cancer. Recognize importance of insurance navigation resources As noted above, I orignally pursued enrolling in Medicare to increase likelihood of reimbursement for proton therapy. I was extremely fortunate to be recommended to an excellent Medicare guide shortly after I was diagnosed with cancer. He recommended a Medigap G plan for my Medicare Part C. The Medigap coverage adds a monthly premium (in total less than my previous private insurance) - however, along with Part A, B and a Part D drug plan - my medical costs for my EV/pembro treatment have been very manageable. As described in my first blog posts, in 2017, I began a (first) health journey, losing 100 lbs. and reversing type 2 diabetes, and after 30 years, began e-biking (I ride the beautiful hills of East Tennessee). I joined the Great Cycle Challenge, where tens of thousands of bikers spend a month riding to raise research funds for children’s cancers. I am riding this month for the seventh year. Although it is devastating to children and their families, due to relatively smaller numbers receives only 4% of federal research funds. Bladder cancer receives roughly 1% of federal funds. So, like the Bladder Cancer Advocacy Network (BCAN), this activity provides young investigators with the funds they need to advance their ideas that may lead to practice-changing breakthroughs such as that I am benefiting from. Data shows the benefits of exercise for cancer patients, and I continue to bike year-round during my journey.
BCAN discusses exercise and cancer here. You can find my Great Cycle Challenge challenge story here I had a genetic test done after I began my treatment (enfortumab vedotin + pembrolizumab / Padcev+Keytruda). This treatment performed so well in its phase 3 trial that no pre-testing (e.g. for PD-1 ) is required for patients presenting with locally advanced or metastatic muscle invasive bladder cancer.
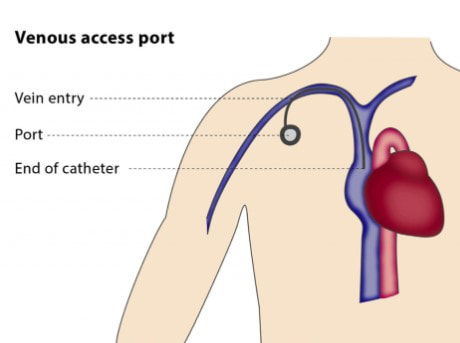
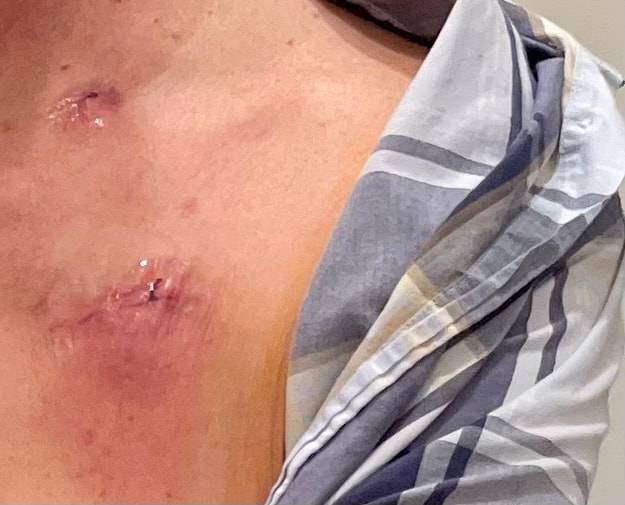
I did request genetic testing where I found I had a PD-L1 CPS score of 10, which indicated that Keytruda should be effective (there is currently no approved biomarker to predict effectiveness of Padcev). I also had germline testing. This is a genetic test that looks for inherited mutations in your DNA that may increase your risk of developing certain types of cancer. These mutations are present in virtually all cells of your body and can be passed down from one generation to the next. I wanted my daughter to be aware of any mutation that might increase her cancer risk. The test found one variation in the CHEK2 gene. When the results came in I met with a genetic counselor who told me that the most recent data suggest a possible increase in breask cancer risk - but that the probability does not warrant any more than the screening which is currently recommended for all women. Germline testing for cancer patients is currently underutilized - I am glad, for my daughter's sake that my oncologist suggested it. On Monday, July 29, I had an outpatient procedure at my primary provider - the University of Tennessee Medical Center - to implant a chemo port. It was my first opportunity to see, up close, interventional radiology equipment from my old employer Siemens. I didn't see it long - I remember being wheeled into the room and then waking up in the recovery room. It was not intubated and happy to be under moderate sedation. A Tylenol the first night after surgery was the only medication I needed. The picture above was from the following morning. I need to keep the incisions dry for a week. The surgical glue will flake off and the sutures will dissolve in time - I need to be the incisions dry for a week.
I was curious and a bit anxious about my next infusion just 4 days after the the implantation surgery. On check-in I said that i had a port installed on Monday. I recognized the nurse who would be performing my first insertion. She carefully organized a set of packets of items which would be required - including masks for both of us. She prepared the area with sanitizer, brought over the access needle, asked me to take a deep breath and then applied the needle. The prick was quick and certainly no worse than the arm poke typically done for lab work. After flushes to ensure that the line was clear, blood for this today's lab work was drawn. The access needle and catheter were left in place and secured with an adhesive patch - the connection would also be used to deliver the infusion. We met with my doctor and then headed upstairs to the infusion suite. My nurse was happy to hear that I had a port, and when my Keytruda was ready, the previously prepared line was connected. No search for a patent IV vein! When the infusion was complete, the line was again flushed and I was on my way. I expect that at my next infusion in 3 weeks, the implantation incisions will be healed. I may look into a getting a lidocain cream that some folks apply about an hour before the access needle is pushed in - but I am not sure that it's really necessary for me. As I am looking at continuing Keytruda for another year and a half - I believe that getting the chemo port was a very good decision. Despite the positive results I have seen with my treatment, I still hesitated before pressing the button to download the notes from today's CT scan with contrast and hesitated again to read through it. Seeing the conclusion brought great relief and a prayer of thanks for God's continuing grace.
Since suspending my enfortumab vedotin (Padcev) - my peripheral neuropathy (numbness in fingers and toes) is subsiding, my sense of taste in normalizing and hair is returning. I am continuing to receive immunotherapy - pembrolizumab (Keytruda) once every 3 weeks. I began infusions (Padcev+Keytruda on day 1, Padcev on day 7 and repeat on day 21) in December. I have three tubes of blood drawn before every treatment to get lab results which are reviewed before treatment. On the last three visits, what used to be one stick to start the treatment IV has been turning into two misses - different locations on the left or right arm or hand. The nurses then go in search of the most experienced person on the floor to put in the IV. I don't suffer from an abject fear of needles (tyrpanophobia), but I will say that I don't look when they are being put in. However, I do feel like I am wearing out my veins and have scheduled an outpatient surgery procedure on July 29 (before my next infusion on August 2) to put in a chemo port. Folks I've asked ovewhelmingly agree with the choice. On the day I departed to Chicago to attend my first annual ASCO meeting as a patient advocate (see previous post), I drew blood for a second circulating tumor DNA test. Once again, no evidence of molecular, residual disease was detected.
After 6 cycles with both pharmaceuticals I began to experience low grade peripheral neuropathy (numbness in fingers and toes) and dysgeusia (taste change). We took a brief "holiday" with two cycles of Keytruda alone. My adverse effects began to subside and my hair began to return. I then had one cycle both at a reduced dose of Padcev. With a second negative ctDNA test and previous positve results reported in earlier posts - we agreed in consultation with my care team to suspend Padcev and maintain Keytruda. We also agreed to continue to keep my bladder (no radical cystectomy or chemo-radiation) and monitor status with periodic ctDNA and imaging. I am interested in collecting data from patients who have experienced a complete pathological response to Padcev+Keytruda treatment after TURBT (prior to radical cystectomy or chemo/radiation) to inform a safe dose de-escalation strategy. I am also interested in gathering from any patients who have used fasting to avoid adverse effects with Padcev+Keytruda treatment. |
AuthorI began a health journey in the fall of 2017 - losing 100 lbs and reversing type II diabetes. Archives
March 2025
Categories |






 RSS Feed
RSS Feed