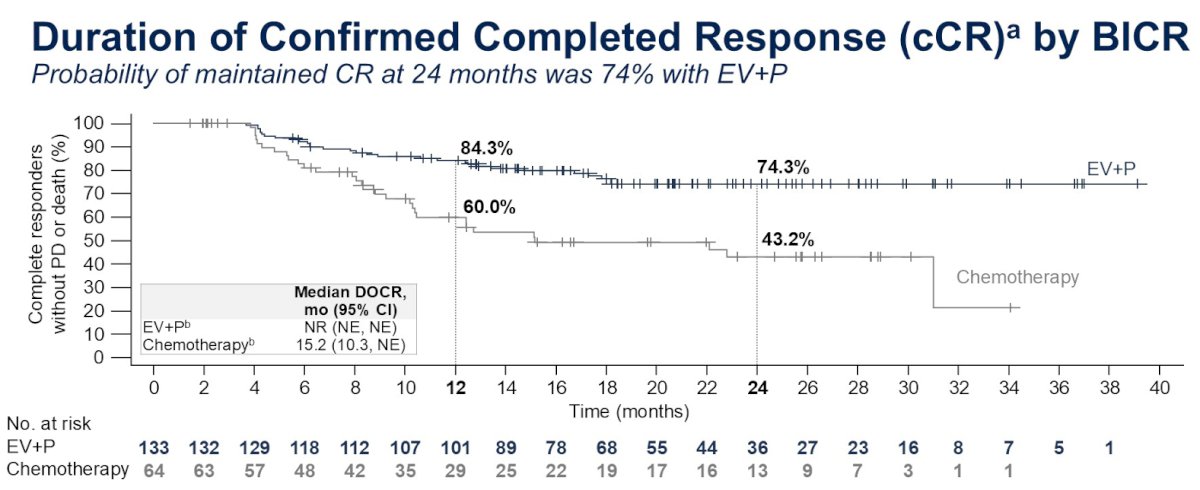
 A Cure Is Possible One of the guidelines of ASCO presentations is to begin by listing the key takeaway points and adding a lay language summary slide at the end. My lens and meeting agenda was influenced by my cancer journey and I begin with my key takeaway. Dr. Jacqueline T. Brown, in a talk entitled "Breaking the Platinum Ceiling in Advanced Bladder Cancer", told of a patient Dan who experienced, as I have, a complete response to treatment with enfortumab vedotin and pembrolizumab and posed a question - essentially one I have posed on this blog before - "Can I be cured". She based her answer on a figure I've displayed before and is now in press. For the EV-302 trial, for those participants achieving a complete response (~29%) - roughly 3 of 4 maintained CR at 2 years. The trend after two years prompted her to annotate the graph and extrapolate out to 5 and even 10 years and tell Dan that a cure is possible. In a post last October, I highlighted a video by Dr. Shilpa Gupta, who commented then that she believed that with EV/pembro, the future could be bladder sparing for many patients with locally advanced MIBC. Dr. Gupta has been a champion for EV/pembro (Dr. Brown had a graphic of Dr. Thomas Powles in a relay race handing the baton to Dr. Gupta). Dr. Gupta had a presentation in the same session and a poster examining the influence of FGFR3 status on immune checkpoint inhibitors in metastatic MIBC. I don't have significant FGRF3 expression. However, the authors stand by their posters during specific hours and I loitered about long enough (many folks were wanting to talk to her) to express my thanks as a complete responder. She was genuinely touched and shared that news like this is what makes the effort worthwhile. I told her a bit about my history and she confirmed Dr. Brown's conclusion. She was curious who my oncologist is. I told her and she quickly asked whether I would mind if we could use her phone to take at selfie together so she could send it to him. Click and off it went - I'm looking forward to seeing it at my next pembro treatment. Only at ASCO! I will keep adding to this post but in the spirit of ASCO wanted to get my first takeaway out.
0 Comments
May is Bladder Cancer Awareness Month. The Bladder Cancer Advocacy Network (BCAN) sponsors a Walk to End Bladder Cancer in a number of cities or a virtual option. I did my level best to issue invitations, on various social media venues I use to connect with fellow bladder cancer survivors, to meet for a walk on May 17th at the new Aspire park.
I put on my BCAN 2025 Walk to End Bladder Cancer t-shirt, BCAN baseball cap, packed my BCAN sling bag, filled BCAN water bottles for my wife and I, picked up my box of BCAN orange bags I had packed with information booklets and bracelets and headed to the park on a gorgeous East Tennessee day - 79 F and light breeze. In the Knoxville area, it’s not uncommon to see orange t-shirts, but nonetheless I had a flash of hope seeing orange clad visitors on the patio outside the delightful Pearl restaurant as we pulled into the parking lot. About the time I verified that it was, naturally, Tennessee Volunteer orange I spied, my phone buzzed to indicate that a family we knew was waiting for us. My wife and I connected with a fine couple through our church’s Cancer Companions ministry. Their kids, whom we had heard much about but not yet met, were thoroughly enjoying the play area, showing their gymnastics skills and seeking to take perfect pictures. As we began to explore the park, the girls ran to joyfully embrace a friend who happened to be enjoying the park with her mom. My wife’s knee has been acting up and after a while she needed to take a seat on the patio. We thanked our friends for coming out and they returned to the kids area. It was a walk after all, and after receiving my wife’s kind permission, I set off to put in a couple more miles and display my bladder cancer awareness message to hikers and bikers enjoying the nicely paved wooded riverside walk and bikeway. As I headed back, I pulled my phone from my pocket, connected via charging cord to a charging pack in my sling and I inquired via text if my wife was interested in getting a cocktail at the restaurant. She coyly replied “perhaps”. As I approached the Pearl, I was greeted by her beautiful smile, as she meditated in a semi-shaded comfy seat overlooking the grounds. We took two chairs and I placed my undistributed gift bags on the bar. We ordered two of an East Tennessee version of a Wisconsin classic - a literally smoked before us Red Maple Old Fashioned (bourbon rather than brandy). As we chatted, a gentleman came up beside me and inquired - “Jon Treffert?”. As I turned around I recognized him immediately as a member of a software team I had led. A fine engineer and a great guy. As we caught up I learned that he was on his own, but different cancer journey. By appearance and attitude, like myself, he was winning his fight. His wife joined us and we reminisced of a gathering at our home (that which we lost to fire two years ago). Like us, their faith was a key component of maintaining hope on their walk. I reflected on the privilege I have had to lead teams dedicated to developing new medical devices to find and defeat cancer. I reflected on the love for my wife and I that would have gone unrecognized had we not faced such trials as losing our home or my cancer diagnosis. I reflected on the fellow survivors, caregivers, physicians and researchers I’ve come to know through BCAN and other outlets. I reflected on the precious gift of the love of my wife and daughter. I still dream of adding Knoxville to the list of cities holding a Walk to end Bladder Cancer. The attendance next year can only get better. I sincerely thank all who have given or may yet give to my walk here Was it worth it? What do you think? 19 months ago I began a journey with a suspicion by CT, observation by cystoscopy and confirmation after transurethral resection of bladder tumor (TURBT) and PET/CT imaging of locally advanced muscle invasive bladder cancer. 16 months ago I began treatment with enfortumab vedotin and pembrolizumab (EV/pembro - Padcev/Keytruda). I currently have no evidence of disease (NED) by circulating tumor DNA (tumor informed Signatera and tumor naive Response). My next surveillance CT, scheduled for May 13, will determine if this trend continues by imaging as well. If I remain NED, my current treatment (pembrolizumab only) will be suspended this Christmas (two years).
In the EV 302 trial, 29% of participants receiving EV/pembro had a confirmed complete response (cCR). Dr. Thomas Powles presented an updated analysis from the EV302 trial at the ASCO GU 2025 in February - including data on the duration of cCR by blinded independent central review (BICR) of imaging data. The performance is sufficiently good that there hasn't been enough time to follow patients to estimate a median cCR duration. At 16 months from beginning treatment about 80% of those achieving cCR had maintained it. The EV302 results look encouraging if I remain NED through Christmas (24 months)... I listened recently to a plenary session of great interest to me at the 40th annual European Association of Urology congress. [I appreciate the work of the EAU and joined as a patient advocate member, in part to access such content.] The topic was bladder sparing strategies - in particular, in cases where a complete response is observed after transurethral resection of the bladder tumor (TURBT) and neoadjuvant systemic therapy- Is active surveillance a safe option? As this is my strategy - my interest was piqued. The discussion was framed around a case study with localized muscular invasive bladder cancer (MIBC). Unlike my case - in this case PET/CT found no spread of disease into local lymph nodes - so the context of the discussion was localised MIBC. However with current treatments and diagnostics, I believe the discussion is applicable to my case of locally advanced MIBC. Dr. Black posed the question: Do we have tools to determine a complete response? One of the studies cited (RETAIN 1) I had mentioned an earlier post. Patients selected for this trial presented with at least one of a set of genetic mutations - including ERCC2. The neoadjuvant chemotherapy was AMVAC . [This study was initiated before the approval of my treatment - enfortumab vedotin and pembrolizumab (EV+pembro).] Active surveillance included imaging but the study was done prior to the emergence of circulating tumor DNA (ctDNA) which I rely on. I had a low mutation burden based on genetic analysis of my tumor, but ERRC2 was found as a likely pathogenic variant. ERCC2 is associated with sensitivity to treatment with cisplatin-based chemotherapy but recently has also been shown to be an independent predictor of favorable progonosis for bladder cancer. I also had a CPS score of 10 for PD-L1. A score of 10 or greater suggest a higher likelihod of benefit from pembrolizumab. However, there is currently no patient selection for EV+pembro by biomarker as none have been shown to be sufficiently predictive of positive response - EV+pembro is approved as an "all comers" first line option for patients with locally advanced or metastatic muscle invasive bladder cancer. In the RETAIN 1 trial, all patients chose to try and preserve their bladder - 48% of the patients who had a complete response remained metatasis-free with intact bladder. In the EV 302 trial which led to approval of my treatment - 38% of the participants had a complete pathological response. The literature does not cite how many (if any) of those patients retained their bladder - but Dr. Black expected that with the advent of new systemic treatments like EV+pembro, more patients (like me) with a complete pathological response, may choose to maintain their bladder and begin active surveillance after systemic treatment. Dr. Black listed a number of tools for determining complete response (see Figure 2). In addition to my toolset, MRI was mentioned - multiparmetric MRI (mpMRI) and new VI-RAD protocools in particular. The first talk in the session presented recent evidence of accuracy of MR for local staging and detecting local recurrence. I lead software development for the first generations of PET/CT and I have confidence PET/CT to detect metastatic disease (it found spread to my pelvic lymph nodes), but I believe my first indication of recurrence will be from ctDNA. Urine circulating tumor DNA was also mentioned but its effectiveness is still being assessed. It was noted that patients (as in the RETAIN 1 trial) who experience recurrence can still undergo radical cystectomy or radiation or potentially second line systemic treatments (see Figure 1). The survival with recurrence in the RETAIN 1 trial was similar to other trials where patients underwent radical cystectomy or trimodality therapy immediately after neoadjuvant systemic therapy. Of course, my hope is that, with EV+pembro, complete pathological response proves to be durable and more patients will elect experiencing it will choose to retain their bladder. The next presentation argued for this strategy- first presenting motivation based on an analysis patient concerns {see Figure 3) compilied in a study from the Bladder Cancer Advocacy Network (BCAN). Dr. Porten based her recommendations (see Figure 4) based on recent trials and indicated possibilities of pursuing them in the follow up to the EV302 trial currently in progress. The next two speakers presented the more common choices (and standard of care if disease has not spread outside the bladder) of radical cystectomy or trimodality therapy (TMT) - radiation and chemotherapy. I also lead software development for a proton therapy system and believe, as was presented by Dr. Choudhury (see Figure 5), that evidence is showing that survival with trimodality therapy is not inferior and may actually be slight better than cystectomy. [I was originally planning on trimodality therapy with proton therapy before learning of the results and approval of EV+pembro.] Dr. Gontero presented arguments for the most common choice with MIBC of radical cystectomy. He identified indications which he saw as contraindications to TMT (see Figure 6). He also argued that RC (with adjuvant systemic therapy) was the best option for long term recurrence free survival for localised disease. Certainly there is a larger body of longer term follow up data as this has been the dominant strategy. I am encouraged to see more presentations like these. The trend in the community, with continuing improvements in treatments and surveillance diagnostics, is pointing to more bladder sparing.
In support of building evidence to include bladder sparing following TURBT and complete pathological response to neoadjuvant treatment, I would like to see the creation of a registry of data of patients who have followed this strategy - more details in my next post... With my most recent CT scan continuing to show no sign of progression and my circulating tumor DNA tests showing no evidence of disease, my oncologist informed me that my Keytruda (pembrolizumab) infusions can stop at 36 (2 years). This first 7 infusions were part of 7 cycles of Padcev (enfortumab vedotin - 14 infusions) and Keytruda. This duration was determined in the KEYNOTE trials of Keytruda with various cancers including advanced urothelial cell carcinoma.
I began treatment on Dec 11, 2023 - 36 Keytruda infusions brings me to Dec 19, 2025. A nurse in the infusion suite said that they will miss me but will celebrate that day with me. Here's to a Merry Christmas after 14 more sessions in the chair.
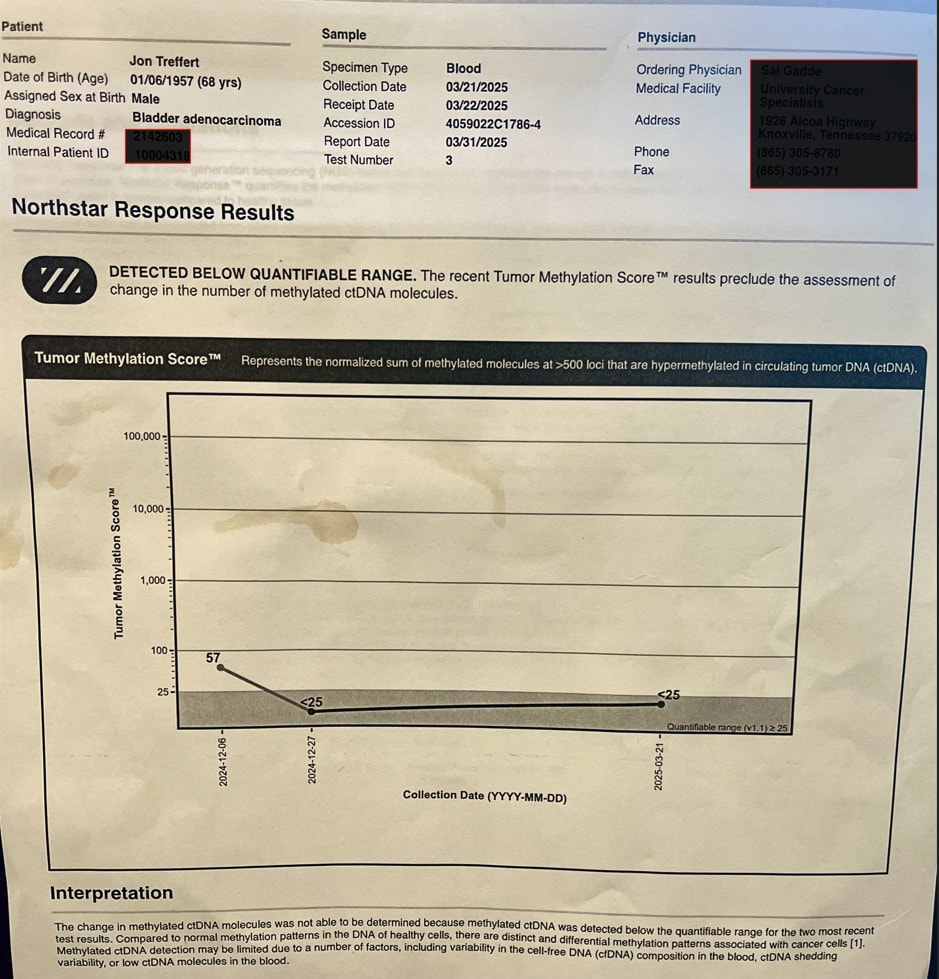
Blood drawn for laboratory work before my last infusion included samples for two circulating tumor DNA (ctDNA) tests - Natera Signatera and Northstar Response (discussed in my previous blog entry).
This is the fourth Signatera test (one roughly every 3 months ) that did not detect ctDNA. The Signatera test is based on genetic analysis of my tumor - looking for the DNA signature of my tumor and 16 likely mutations. The Response test looks for methylated DNA - a byproduct of cancer at work. It is estimated to have 10 times the "signal" of Signatera. My oncologist indicated that, because of it's sensitivity, he has seen changes even between cycles (every 3 weeks for me). I was encouraged to see that my second Response value was less than 25 and below its quantifiable range. This was not considered a significant change from my baseline (57). I will discuss with oncologist if I should draw blood for another Response test at my next infusion. I've also had a PET/CT scan which indicated that my lymph nodes were no longer active and two subsequent CT scans (all roughly 3 months apart) which showed no evidence of new metastatic disease. This surveillance data suggests to me I have had a complete pathological response (pCR) - as did 29% of participants in the EV302 trial, which led to approval of my treatment (enfortumab vedotin+pembrolizumab) as a first line option. I suspended enfortumab vedotin (Padcev) after 7 cycles due to adverse effects described previously. I've continued pembrolizumab (Keytruda) - 11 additional cycles so far. With my second Response test being consistent with my Signatera test - it raises the question of when I can safely suspend my Keytruda. pCR unfortunately does not mean that cancer is gone or will not recur. A recent article suggests that adjuvant immunotherapy should not be given to patients who exhibit pCR after neoadjuvant chemoimmunotherapy and surgery. The context is patients with resectable non-small cell lung cancer (NSCLC) - who receive a combination of immunotherapy and platinum-based chemo before surgery and demonstrate pCR after surgery. Results of recent trials suggest that pCR has a prognostic value for high event free survival (EFS). The authors note that the majority of patients (93% in the CheckMate-816 trial) who achieved pCR had no detectable ctDNA. They point out that an additional year of immunotherapy comes with risks of adverse effects and a financial cost (to insurer or patient). Given the encouraging results for patients who achieve pCR - they don't see the benefits outweighing risks in follow up immunotherapy. In my case surgery was my initial transurethral resection of the bladder tumor (TURBT) and chemoimmunotherapy was Padcev+Keytruda. My adjuvant immunotherapy is continuation of Keytruda. I believe that I have achieved pCR and will seek forums to pose the question (including discussing with my oncologist) of how long I should continue my immunotherapy and monitor the literature for any related guidance about continuing immunotherapy (unless and until I see recurrence of my disease). It seems there is a name for approach I have been pursuing (and providentially led to) - risk adapted treatment for locally advanced muscle invasive bladder cancer: employing a systemic treatment - in my case enfortumab vedotin + pembrolizumab (EV/pembro or Padcev/Keytruda) - and active surveillance to determine if other treatment options are needed - which could include bladder removal (radical cystectomy) or chemoradiation (trimodality therapy). The results of a phase 2 trial following this approach were just published. The RETAIN-1 (NCT02710734) trial began 2016, before EV/Pembro was approved, and the neoadjuvant systemic therapy was AMVAC (accelerated methotraxate, vinblastine, doxorubicin and cisplatin). Genetic analysis was done on the tumors after transurethral resection of the bladder tumor (TURBT) looking for ATM, RB1, FANCC or ERCC2 mutations. After 3 cycles of AMVAC were completed and a second TURBT performed, patients with no residual disease and at least one of the listed mutations began active surveillance (AS). The end point of the trial was to be metastasis free survival IMFS) at 2 years for all enrolled. The end point was not met, however of the 25 patients enrolled in AS, 12 (48%) remained metastasis free with intact bladders at 2 years.
I began treatment with EV/pembro a bit more than a year ago. EV/pembro is an "all comers" treatment. EV delivers a chemothearpy payload to a Nectin-4 protein expressed on the majority of urothelial cancer cells and pembrolizumab blocks the PD-1 protein on immune cells to energize the immune system. Participants in the EV302 trial that led to EV/pembro approval were tested for PD-1 and Nectin-4. There was success (and some failures) for those with both high and low expression of both biomarkers. Therefore there is, at present, no biomarker to reliably select patients likely to have a positive response (which included a complete response for 29% of the participants in EV302). After 4 cycles of treatment, I had my first indication that I no longer had evidence of disease by PET/CT imaging and tumor-informed circulating tumor DNA (ctDNA). I've had two other surveillance data points, each roughly 3 months apart - CT scans showing no new metastases and no detected ctDNA by Signatera test. We suspended Padcev after 7 cycles and have continued with Keytruda. My oncologist told me of another ctDNA test - Northstar Response - which is tumor naive (not informed by analysis of my tumor). It looks for methylated tumor DNA - a byproduct of cancer at work - shed in the blood. The signal is potentially much higher and my oncologist has seen changes even between cycles (at the moment every 3 weeks for pembrolizumab alone). I drew blood for my first Northstart Response test on December 6 - the results are shown above. Not what I was hoping for, my baseline result indicates that there is still cancer there - low, but above the level where the value is too low to be certain. I drew blood for my second Response test on December 21 - where we can see if the trend is up, down or stable and plan action accordingly - again risk-adapted treatment. The baseline test also called out a CHEK2 mutation - which was also identified in my germline test (discussed in a previous post). The test also listed related clinical trials. NCT03375307 is clinical trial is a Phase II study evaluating the efficacy of olaparib (a PARP inhibitor) in treating patients with metastatic or advanced urothelial cancer that has DNA-repair defects (including CHEK2 mutation). I am reaching out to the investigators leading this trial to see if and when this might be a second line option if needed (restarting Padcev would be another option). Information is power and I know there is power in prayer and I plan to engage both. The weather, holidays and perhaps a bit of complacence have conspired to slow the pace of my exercise and periodic fasting - along with an increase in my weight and blood glucose. I will attend to these and while I wait on the next ctDNA results - prayers are always welcome. I began treatment for locally advanced muscle invasive bladder cancer on December 15 last year.
Watching my sheep today reminds me of THE gift that secured my eternal destiny in a new creation free of disease. Until then my Christmas list... Complete response to new systemic treatments (e.g. antibody drug conjugates and immunotherapy) for locally advanced muscle invasive bladder cancer and others will be shown to be durable. AI will accelerate development and validation of better treatments. Biomarkers will be developed for accurate patient selection for these treatments. Molecular residual disease tests MRD (e.g circulating tumor DNA) will simplify surveillance and support prompt changes in management - including safe treatment de-escalation. Improved overall survival prognoses with preservation of quality of life (including bladder preservation) will be the norm. My fellow survivors will feel their Savior walking with them. WIshing all a joyful and blessed Christmas! A recent publication caught my eye where a study was done with mouse models of and human patients with prostate cancer.
In the mouse models, the addition of white button mushroom (WBM) extract to the diet resulted in reduction in the number of myeloid-derived suppressor cells (MDSC) which promoted antitumor immune responses mediated by T cells and natural killer (NK) cells. They also observed increased anticancer activity of PD-1 antibodies In patients a decline was observed in the circulating MDSCs and increase in cytotoxic CD8+ T and NK cells. The City of Hope is recruiting for a phase 2 trial to further examine the effect of WBM on prostate cancer. MDSCs are also emerging target for bladder cancer. They contribute to the ability of tumors to evade immune detection. More research, such as that being pursied for prostate cancer, is needed to demonstrate the effect of reducing MDSCs in bladder cancer patients - however, the mechanism is the same for both. White button mushrooms offer a number of health benefits (including gut biome health and glucose control which I have mentioned elsewhere are of interest to me) and WBM extract is available as a supplement. My single treatment for muscle invasive bladder cancer at the moment is pembrolizumab - an immune checkpoint inhibitor. I've discussed with my oncologist and I am going to taking a daily dose of WBM extract to potentially enhance its effect. I will start with dose recommended on the product I ordered (500 mg - roughly 1/4 teaspoon daily). I may adjust as I learn more of the dose used in clinical trials. As always, with any change in diet or medication, you should consult with your care team. |
AuthorI began a health journey in the fall of 2017 - losing 100 lbs and reversing type II diabetes. Archives
March 2025
Categories |






















 RSS Feed
RSS Feed